Silica, chemically known as silicon dioxide (SiO2), is a versatile and widely used material with a multitude of applications. While silica is commonly recognized as an essential component of glass and ceramics, it also plays a crucial role in catalysis. Catalysis is a process that accelerates chemical reactions by lowering the activation energy required for the reaction to occur. In this article, we delve into the comprehensive role of silica as a catalyst, exploring its properties, various forms, and applications across different industries.
- Properties of Silica: Silica possesses several unique properties that make it an ideal material for catalytic applications. These properties include:
- High surface area: Silica-based catalysts typically have high surface areas, providing a large number of active sites for catalytic reactions to take place.
- Thermal stability: Silica exhibits excellent thermal stability, enabling its use in high-temperature catalytic processes.
- Chemical inertness: Silica is chemically inert, ensuring minimal interference with the reactants and products during catalysis.
- Forms of Silica Catalysts: Silica catalysts exist in various forms, each offering specific advantages depending on the desired application. Some common forms of silica catalysts include:
- Silica Gel: Silica gel is a porous and amorphous form of silica. It has a high surface area, making it effective for adsorption and heterogeneous catalysis.
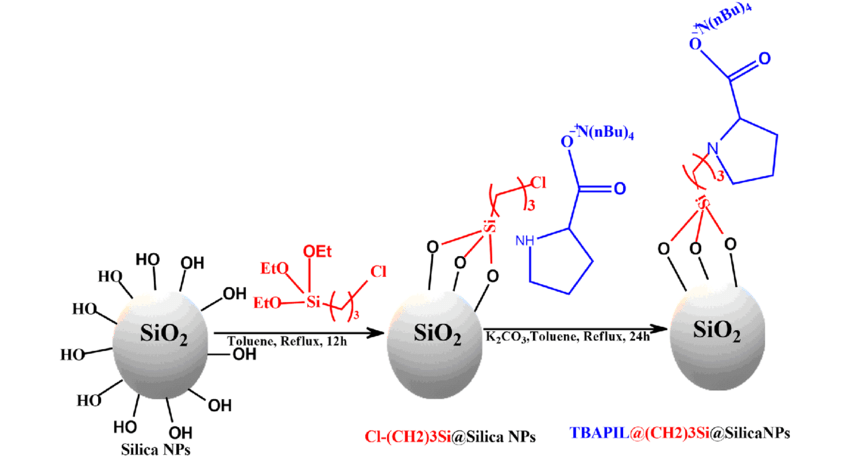
- Silica-supported Catalysts: Silica can serve as a support material for other catalytic species, such as metals or metal oxides. This allows for the enhancement of catalytic activity and selectivity.
- Mesoporous Silica: Mesoporous silica possesses a well-defined network of pores with diameters typically ranging from 2 to 50 nanometers. This form of silica provides controlled porosity, enabling precise control over reactant diffusion and reaction rates.
- Applications of Silica Catalysts: Silica-based catalysts find applications across various industries due to their unique properties. Here are some notable examples:
- Petrochemical Industry: Silica catalysts are widely employed in petroleum refining processes, such as fluid catalytic cracking (FCC). They help in the conversion of heavy hydrocarbon fractions into more valuable light olefins and gasoline components.
- Fine Chemicals and Pharmaceutical Industry: Silica catalysts play a vital role in the synthesis of fine chemicals and pharmaceutical intermediates. They facilitate critical reactions like oxidation, reduction, and rearrangement, enabling the production of complex organic molecules.
- Environmental Applications: Silica catalysts contribute to environmental sustainability by aiding in pollution control. They are used in catalytic converters to convert harmful exhaust gases, such as nitrogen oxides (NOx), into less harmful compounds.
- Polymerization Reactions: Silica-supported catalysts are widely used in polymerization processes, such as the production of polyethylene and polypropylene. These catalysts exhibit excellent control over molecular weight distribution and polymer morphology.
- Mechanisms of Silica Catalysis: The exact mechanisms by which silica catalysts operate vary depending on the specific reaction and catalyst form. However, some general catalytic processes involving silica include:
- Acid-Base Catalysis: Silica possesses Lewis acid and Brønsted acid sites, enabling it to catalyze acid-base reactions. The surface hydroxyl groups on silica act as Brønsted acid sites, while metal impurities or cations present on the surface can act as Lewis acid sites.
- Surface Activation: Silica catalysts can activate certain reactants by adsorbing them onto the catalyst surface, thereby facilitating subsequent reactions.
- Shape Selectivity: The porous nature of silica catalyst, especially mesoporous silica, imparts shape selectivity to the catalytic process. The well-defined pore structure allows for the preferential adsorption and reaction of molecules of specific sizes and shapes, leading to the formation of desired products.
- Stabilization of Reactive Intermediates: Silica can stabilize reactive intermediates formed during catalytic reactions. The surface silanol groups can interact with and stabilize transient species, preventing their decomposition and facilitating subsequent reaction steps.
- Catalyst Design and Modification: The catalytic performance of silica catalysts can be further enhanced through design and modification strategies. Some common techniques include:
- Control of Pore Structure: Tailoring the pore size and distribution of mesoporous silica catalysts allows for improved mass transport and accessibility of reactants, leading to enhanced catalytic activity.
- Introduction of Active Sites: Silica-supported catalysts can be modified by incorporating metal nanoparticles, metal oxides, or other catalytically active species onto the silica surface. These active sites promote specific reactions and improve overall catalytic performance.
- Surface Functionalization: Silica surfaces can be functionalized with organic or inorganic groups to introduce additional catalytic functionalities or to enhance selectivity and stability.
- Challenges and Future Perspectives: While silica catalysts offer numerous advantages, certain challenges remain to be addressed. These include:
- Catalyst Deactivation: Silica catalysts can be prone to deactivation due to factors such as fouling, poisoning, or sintering. Research efforts are focused on developing more stable catalyst formulations and regeneration techniques.
- Selectivity Control: Achieving high selectivity in complex reactions can be challenging. Further research is needed to optimize catalyst design and reaction conditions to achieve desired selectivity while minimizing unwanted side reactions.
- Sustainability and Environmental Impact: The synthesis and disposal of silica catalysts can have environmental implications. Exploring greener synthesis methods and catalyst recycling approaches can contribute to sustainability.
Looking ahead, the future of silica catalysts lies in the development of advanced nanoscale and mesoporous materials, precise control of active sites, and tailored catalysts for specific applications. Integration with other catalytic materials and techniques, such as zeolites or enzyme immobilization, holds potential for further advancements in catalysis.
Conclusion: Silica, with its unique properties and diverse forms, plays a crucial role as a catalyst across various industries. Its high surface area, thermal stability, and shape selectivity make it an effective material for accelerating chemical reactions. From petroleum refining to pharmaceutical synthesis and environmental applications, silica catalysts have proven indispensable. Continued research and innovation in catalyst design and modification will further enhance their performance, contributing to advancements in catalysis and enabling sustainable chemical processes in the future.